Bridging Patients and Researchers to Accelerate Kidney Disease DiscoverY
The Clinical Phenotyping Resource and Biobank Core (C-PROBE) offers an unparalleled, well-annotated resource of clinical data and biospecimens from over 1,700 individuals with chronic kidney disease—spanning both children and adults across multiple clinical sites—collected and followed longitudinally for more than 15 years.
By bridging the gap between patients in clinical care settings and investigators conducting biomedical and translational research, C-PROBE accelerates discovery and fosters innovation in kidney disease research, enabling impactful studies that drive new insights and advance clinical care.


mission
Our primary goal is to develop an infrastructure that serves as an interface between patients in the clinical care settings and biomedical investigators conducting translational research in kidney disease. Multiple specific research studies have now successfully utilized these resources.
C-PROBE aims to:
- Create a collaborative agreement for operating a biomedical research core
- Establish an ethnically diverse pool of human research subjects consisting of individuals with kidney disease
- Develop a streamlined infrastructure for recruitment of human subjects into clinical and translational research protocols using a customized, secure, web-based relational research data management system
- Maintain a centralized bank of biological specimens and clinical data
C-PROBE Data At A Glance
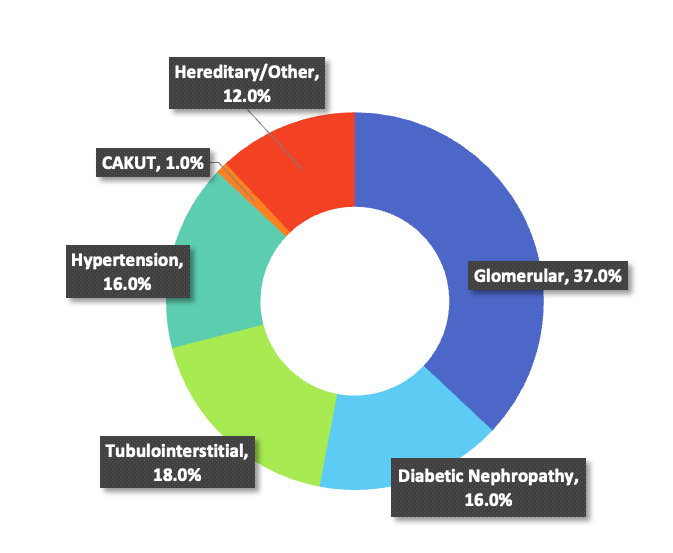
Disease Types
Current disease etiology breakdown is 37% Glomerular, 16% Diabetic Nephropathy, 18% Tubulointerstitial, 16% Hypertension, 1% CAKUT, 12% Hereditary/Other.
Specimens
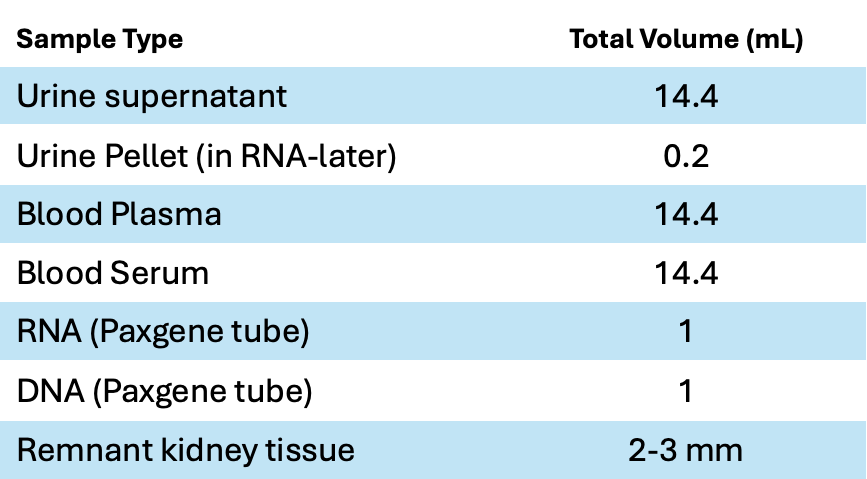
Biological specimens include remnant kidney tissue, blood including plasma (EDTA), serum (SST), DNA and RNA, and urine preserved with both protease inhibitor and sodium azide.
The study has 45,600 plasma and serum samples, 53,400 urine-derived samples, and remnant tissue in RNA-Later from 129 renal biopsies.
Demographics
Demographic data collected annually includes age (at enrollment), annual household income, educational level, race and ethnicity, gender, height and weight, employment status, and primary type of work, among others.
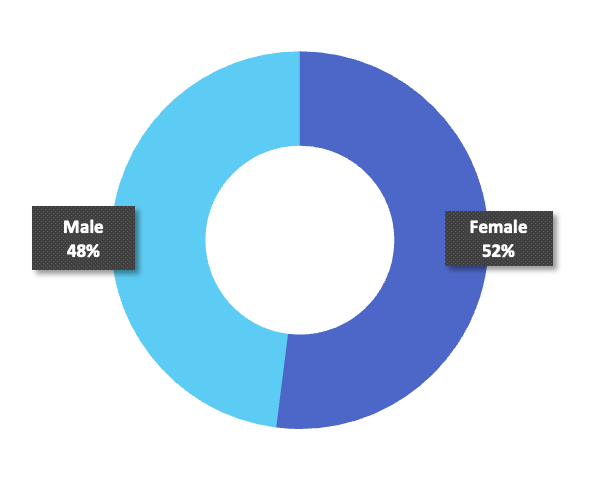
Gender
Gender breakdown is 52% female, 48% male
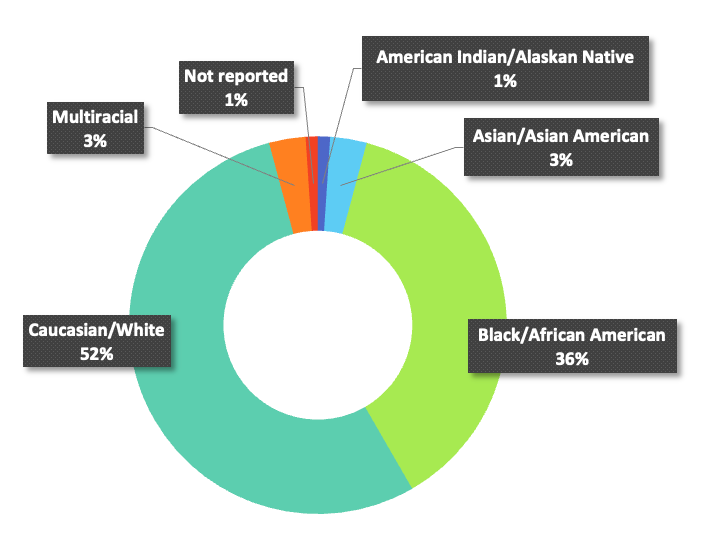
Race
9% Self identify as Hispanic. 33.78% of Black participants experienced 40% or greater decline in eGFR, with 41.18% of white participants having the same decline. All-cause mortality of 11.95% Black and 9.32% white were also observed.
C-Probe Ancillary Studies
C-PROBE welcomes and encourages internal and external investigators to use the observational clinical data, histopathology data, biosamples, whole slide images, and derived data sets from C-PROBE for their own research.
Please submit an ancillary study proposal to the C-PROBE Steering Committee at least 2 months prior to the anticipated external funding submission date. To submit an ancillary study proposal, please contact C-PROBE-support@umich.edu and an ancillary application will be emailed to you.
Once received, the ancillary study proposal will be reviewed by our Steering Committee to ensure that minimal burden on our data support staff and sample procurement from our biorepository while using these resources prudently. We also would like to manage scope overlap for other ongoing ancillary studies and potentially put you in touch with ancillary investigators with collaborative interests.
C-PROBE Leadership

Professor of Internal Medicine and Professor of Molecular and Integrative Physiology
Section Head, Division of Nephrology




& Biomarker Research Lab

